
FDA MedWatch
Exela Pharma Sciences, LLC Issues Voluntary Nationwide Recall of 8.4% Sodium Bicarbonate Injection, USP, 50 mEq/50 mL, Midazolam in 0.8% Sodium Chloride Injection 100 mg/100 mL, and ELCYS (cysteine hydrochloride Injection), USP 500 mg/10 mL Due to the Presence of Particulate Matter
KCER Release Date: October 27, 2023
To: KCER Distribution list- including ESRD Network EDs and QIDs
Summary
Company Announcement Date: October 25, 2023
FDA Publish Date: October 25, 2023
Product Type: Drugs
Reason for Announcement: Potential presence of particulate matter
Company Name: Exela Pharma Sciences, LLC
Brand Name: Exela and Civica Brands
Product Description: Sodium Bicarbonate Injection, USP, Midazolam in 0.8% Sodium Chloride Injection ELCYS (cysteine hydrochloride Injection), USP
Company Announcement
FOR IMMEDIATE RELEASE – October 25, 2023 – Lenoir, North Carolina. Exela Pharma Sciences, LLC, (Exela) is voluntarily recalling the products listed in the table below to the consumer level. Particulate matter identified as silicone was observed during routine inspection of retain samples.
Product
Strength
Vial Size
8.4% Sodium Bicarbonate Injection, USP
50 mEq/50 mL
50 mL Single Dose Vial
Midazolam in 0.8% Sodium Chloride Injection
100 mg/100 mL
100 mL Single Dose Vial
ELCYS (cysteine hydrochloride Injection), USP
500 mg/10 mL
10 mL Single Dose Vial
Risk Statement: Administration of an injectable product that contains particulate matter may result in local irritation or swelling in response to the foreign material. If the particulate matter reaches the blood vessels it can travel to various organs and block blood vessels in the heart, lungs or brain which can cause stroke and even lead to death. Exela has not received any reports of adverse events related to this recall.
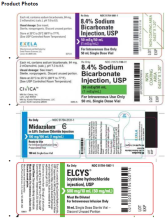
8.4% Sodium Bicarbonate Injection USP is used for treatment of metabolic acidosis and is packaged in a 50 mL glass single dose vials, 20 vials per carton Exela brand (Carton NDC: 51754-5001-5; Vial NDC: 51754-5001-1, Figure 1) and 25 vials per carton Exela brand (Carton NDC: 51754-5001-4; Vial NDC: 51754-5001-1) and Civica brand (Carton NDC: 72572-740-20; Vial NDC: 72572-740-01, Figure 2).
The affected 8.4% Sodium Bicarbonate Injection, USP, 50 mEq/50 mL lots (covering both Exela and Civica brands) include the following lot numbers and expiration dates:
Brand
Lot
Expire Date
Exela
P0001429
11/2023
Exela
P0001900
08/2024
Exela
P0001902
08/2024
Exela
P0001903
09/2024
Exela
P0001909
09/2024
Civica
P0001912
08/2024
Exela
P0001945
09/2024
Exela
P0002002
11/2024
Exela
P0002052
12/2024
Product was distributed nationwide to wholesalers, distributors, and health systems between January 18, 2022 and February 15, 2023.
Midazolam in 0.8% Sodium Chloride Injection is used for sedation and is packaged in a 100 mL glass vial, 25 vials per corrugated shipper. The vials are labeled with Exela brand (Carton NDC: 51754-2131- 4; Vial NDC: 51754-2131-1, Figure 3).
The affected Midazolam in 0.8% Sodium Chloride Injection 100 mg/ 100 mL include the following lot number and expiration date:
Brand
Lot
Expire Date
Exela
10001088
07/2024
Product was distributed nationwide to wholesalers, distributors, and health systems between July 14, 2023, and September 26, 2023.
ELCYS (cysteine hydrochloride Injection) is used for nutritional requirements per total parenteral nutrition (TPN) and is packaged in a 10 mL glass vial, 10 vials per carton. The vials are labeled with Exela brand (Carton NDC: 51754-1007-3; Vial NDC: 51754-1007-1, Figure 4).
The affected ELCYS (cysteine hydrochloride Injection), USP 50 mg/mL includes the following lot number and expiration date:
Brand
Lot
Expire Date
Exela
10000798
03/2025
Product was distributed nationwide to wholesalers, distributors, health systems, and compounders between July 20, 2023, and August 1, 2023.
Exela is notifying its customers by e-mail and certified mail and is arranging for return and replacement of all recalled product directly to Exela. Customers that have product which is being recalled should discontinue use, segregate the recalled product, submit a recall stock response form to Exela (even if there is no product to return), and hold the product until shipment instructions are provided by Exela.
Customers with questions regarding this recall can contact Exela by phone (828-341-6118) or email at recall@exela.us Monday through Friday, 9:00am – 5:00pm ET. Consumers should contact their physician or healthcare provider if they have experienced any problems related to the usage of this drug product.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being executed with the knowledge of the U.S. Food and Drug Administration.
Company Contact Information
Consumers:
Exela
828-341-6118
Product Photos
